Causes of Corrosion of SS Flanges in a Nuclear Power Plant (Part One)
Overview
The important water system (SEC) is an open circulation system; its internal medium is seawater, and its main function is to cool the cooling system of the cooling equipment. The main function of RRI is to cool various types of heat exchangers in the nuclear island. The heat load of the nuclear island user collected by the RRI system is transported to the final heat sink, that is, the sea. Each unit has two safety related redundant series, and the SEC system is a nuclear safety-related system. In order to monitor the upstream and downstream of the plate heat exchanger, the fluid pressure is measured, and a branch pipe is arranged at the inlet pipe position of the plate heat exchanger to measure the pressure in the pipe.
The design material of the plate heat exchanger's inlet pipe is P265GH, and the diameter is 711.2mm; the internal anti-corrosion adopts neoprene rubber with a thickness of 4mm. The material and anti-corrosion design of the branch pipe are the same as those of the inlet pipe, with a diameter of 88.9mm, and the branch pipe and the inlet pipe form an angle of 45. The material of the flange of the differential pressure gauge is Z2CND1712, which is similar to 316L. The flange and the branch pipe are connected by bolts, and the gasket is a rubber gasket. The schematic diagram of the pipeline layout is shown in Figure 1.
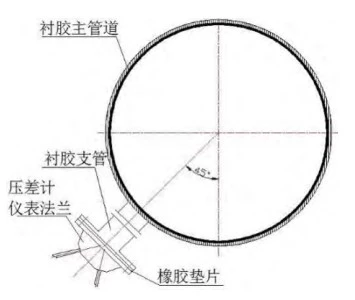
Figure 1 The layout structure of the pipeline
In July 2016, the corrosion inspection of the stainless steel flange of the SEC system was carried out. It was found that there were crescent-shaped corrosion pits and pitting pits in the joint surface of the stainless steel flange and the rubber-lined branch pipe. Corrosion products are removed after grinding, and it was found that the deepest corrosion pit was about 2mm, as shown in figure 2.

Figure 2 The morphology of corrosion pits of stainless steel flanges
1. Analysis of the causes of corrosion
1.1 Analysis of corrosion resistance of stainless steel
The reason why stainless steel can be stainless is that a passivation film is formed on its surface, which can improve the corrosion resistance on the surface of the metal. However, this stainless is only relative. Under normal circumstances, the passivation film is in a stable state and can repair itself, but some ions dissolve the passivation film in a specific environment. If the dissolution rate of the passivation film is greater than its repair speed, corrosion occurs on the surface of stainless steel.
Different types of stainless steel have different corrosion resistance. After long-term research on the corrosion of stainless steel by researchers, it is found that there is a certain relationship between the composition of stainless steel and the comprehensive corrosion resistance. If the influence of the corrosive medium is not considered, the corrosion resistance of stainless steel can be quantitatively indicated by the calculated value, which is PRE (Pitting Resistance Equivalent), and the calculation formula is as follows:
PRE=Cr+3.3Mo+16N
The chemical elements Cr, Mo, and N in the formula represent their percentage content. PRE is a pitting resistance equivalent, which can be used as a method of showing the general comprehensive corrosion resistance of stainless steel. PRE can be used to compare the corrosion resistance of stainless steel with different grades. The greater the PRE value is, the better the corrosion resistance of the stainless steel becomes.
1.2 Common stainless steel corrosion mechanism in seawater
Stainless steel is increasingly used in marine environments, such as valves, pipes, pump casings and propellers due to its good corrosion resistance. Although stainless steel has good corrosion resistance, it will still corrode in certain marine environments, and this corrosion is mainly partial corrosion, such as pitting corrosion, crevice corrosion, etc.
(1) Pitting
If most of the surface of the metal does not corrode, but only partial corrosion holes appear and develop in depth and breadth, this is called pitting corrosion. In seawater, the pitting of stainless steel includes pitting, groove corrosion, and tunnel corrosion. The formation of pitting pits in seawater is divided into two processes: the formation of corrosion nuclei and the development of pitting pits.
The formation of corrosion nuclei is because the chloride ions in seawater preferentially adsorb the stainless steel passivation film, squeeze the oxygen atoms, and then combine with the cations in the passivation film to form soluble oxides, which finally form corrosion nuclei on specific points of the newly exposed base metal. If the passivation happens again at the nucleation position and resistance of the metal is great at this time, the nucleation will continue to grow. When the nucleation grows to a certain extent, a pitting hole will be formed.
The development of pitting is mainly driven by the formation of occluded cells inside and outside the corrosion hole, that is, the dissolution of anode metal inside the pit (Fe→Fe2++2e), and the reduction reaction of cathodic oxygen outside the pit (O+ 2H2O+4e- →4(OH)-). As the reaction continues, positive ions in the etch pits gradually increase. Chloride ions are attracted by positive ions and move into the pit to form ferrous chloride to maintain electrical neutrality, and ferrous chloride is hydrolyzed (FeCl2+2H2O→Fe (OH)2+2HCI) to acidify the solution in the pit. In addition, corrosion products accumulate at the mouth of the etching pit, hindering convection and oxygen diffusion, so that the solution in the hole cannot be diluted and the metal's passivation cannot obtain enough oxygen. The decrease of pH value and oxygen content in the pit accelerates the dissolution of metals in the pit, which in turn promotes the inhalation and hydrolysis of CI ions. The above process repeats itself to form the autocatalytic process of the closed battery, and it is also the process that the metal in the pit is continuously dissolved. As shown in Figure 3.
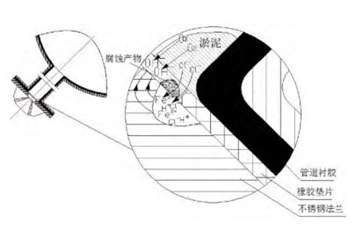
Figure 3 The principle of pitting
(2) Crevice corrosion
Crevice corrosion is partial corrosion caused by the formation of concentration cells in the electrolyte, where the movement of related substances is blocked in very narrow gaps between surfaces of metals or non-metals. Crevice corrosion is a relatively common partial corrosion. For almost all metals and all media, if there is a stagnation gap, there will be crevice corrosion; passive metals are especially sensitive in neutral media containing active anions (CI and FR). The development process of crevice corrosion includes the lack of oxygen in the crevice, the hydrolysis of metal ions, the acidification of the solution, the destruction of the passivation film, the increase of the metal dissolution rate, and the autocatalytic process.
The principle of crevice corrosion is similar to that of pitting corrosion, both of which are caused by the autocatalytic effect of occluded cells. The oxygen content in the gap cannot be replenished in time due to the narrow gap, coupled with the migration of chloride ions and the hydrolysis of chloride; the pH value of the solution in the gap decreases, which further accelerates the dissolution of metals in the gap, as shown in Figure 4.
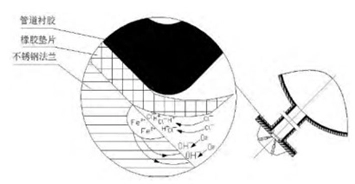
Figure 4 The principle of crevice corrosion
(3) Corrosion of oxygen concentration cells
Corrosion of oxygen concentration cells refers to a kind of corrosion behavior formed by the difference in oxygen. In an oxygen concentration cell, the region with a higher oxygen concentration on the metal surface has a higher potential and becomes the cathode of the corrosion cell. On the contrary, the region with a lower oxygen concentration on the metal surface has a lower potential and becomes the anode of the corrosion cell. The Nester equation describing the equilibrium potential in thermodynamics is used to explain the difference in potential when the metal is in contact with different concentrations of oxygen. The metal potential Ee1 in the oxygen-deficient region is lower, which becomes the anode region. The metal potential Ee2 in the oxygen-rich region is higher, which becomes the cathode region, as shown in Figure 5 (ignoring the ohmic potential drop in the solution).
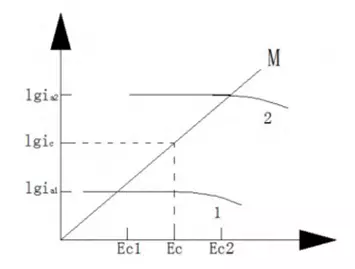
Figure 5 Corrosion of metals in oxygen-deficient zone 1 and oxygen-enriched zone 2
The important water system (SEC) is an open circulation system; its internal medium is seawater, and its main function is to cool the cooling system of the cooling equipment. The main function of RRI is to cool various types of heat exchangers in the nuclear island. The heat load of the nuclear island user collected by the RRI system is transported to the final heat sink, that is, the sea. Each unit has two safety related redundant series, and the SEC system is a nuclear safety-related system. In order to monitor the upstream and downstream of the plate heat exchanger, the fluid pressure is measured, and a branch pipe is arranged at the inlet pipe position of the plate heat exchanger to measure the pressure in the pipe.
The design material of the plate heat exchanger's inlet pipe is P265GH, and the diameter is 711.2mm; the internal anti-corrosion adopts neoprene rubber with a thickness of 4mm. The material and anti-corrosion design of the branch pipe are the same as those of the inlet pipe, with a diameter of 88.9mm, and the branch pipe and the inlet pipe form an angle of 45. The material of the flange of the differential pressure gauge is Z2CND1712, which is similar to 316L. The flange and the branch pipe are connected by bolts, and the gasket is a rubber gasket. The schematic diagram of the pipeline layout is shown in Figure 1.

Figure 1 The layout structure of the pipeline
In July 2016, the corrosion inspection of the stainless steel flange of the SEC system was carried out. It was found that there were crescent-shaped corrosion pits and pitting pits in the joint surface of the stainless steel flange and the rubber-lined branch pipe. Corrosion products are removed after grinding, and it was found that the deepest corrosion pit was about 2mm, as shown in figure 2.

Figure 2 The morphology of corrosion pits of stainless steel flanges
1. Analysis of the causes of corrosion
1.1 Analysis of corrosion resistance of stainless steel
The reason why stainless steel can be stainless is that a passivation film is formed on its surface, which can improve the corrosion resistance on the surface of the metal. However, this stainless is only relative. Under normal circumstances, the passivation film is in a stable state and can repair itself, but some ions dissolve the passivation film in a specific environment. If the dissolution rate of the passivation film is greater than its repair speed, corrosion occurs on the surface of stainless steel.
Different types of stainless steel have different corrosion resistance. After long-term research on the corrosion of stainless steel by researchers, it is found that there is a certain relationship between the composition of stainless steel and the comprehensive corrosion resistance. If the influence of the corrosive medium is not considered, the corrosion resistance of stainless steel can be quantitatively indicated by the calculated value, which is PRE (Pitting Resistance Equivalent), and the calculation formula is as follows:
PRE=Cr+3.3Mo+16N
The chemical elements Cr, Mo, and N in the formula represent their percentage content. PRE is a pitting resistance equivalent, which can be used as a method of showing the general comprehensive corrosion resistance of stainless steel. PRE can be used to compare the corrosion resistance of stainless steel with different grades. The greater the PRE value is, the better the corrosion resistance of the stainless steel becomes.
1.2 Common stainless steel corrosion mechanism in seawater
Stainless steel is increasingly used in marine environments, such as valves, pipes, pump casings and propellers due to its good corrosion resistance. Although stainless steel has good corrosion resistance, it will still corrode in certain marine environments, and this corrosion is mainly partial corrosion, such as pitting corrosion, crevice corrosion, etc.
(1) Pitting
If most of the surface of the metal does not corrode, but only partial corrosion holes appear and develop in depth and breadth, this is called pitting corrosion. In seawater, the pitting of stainless steel includes pitting, groove corrosion, and tunnel corrosion. The formation of pitting pits in seawater is divided into two processes: the formation of corrosion nuclei and the development of pitting pits.
The formation of corrosion nuclei is because the chloride ions in seawater preferentially adsorb the stainless steel passivation film, squeeze the oxygen atoms, and then combine with the cations in the passivation film to form soluble oxides, which finally form corrosion nuclei on specific points of the newly exposed base metal. If the passivation happens again at the nucleation position and resistance of the metal is great at this time, the nucleation will continue to grow. When the nucleation grows to a certain extent, a pitting hole will be formed.
The development of pitting is mainly driven by the formation of occluded cells inside and outside the corrosion hole, that is, the dissolution of anode metal inside the pit (Fe→Fe2++2e), and the reduction reaction of cathodic oxygen outside the pit (O+ 2H2O+4e- →4(OH)-). As the reaction continues, positive ions in the etch pits gradually increase. Chloride ions are attracted by positive ions and move into the pit to form ferrous chloride to maintain electrical neutrality, and ferrous chloride is hydrolyzed (FeCl2+2H2O→Fe (OH)2+2HCI) to acidify the solution in the pit. In addition, corrosion products accumulate at the mouth of the etching pit, hindering convection and oxygen diffusion, so that the solution in the hole cannot be diluted and the metal's passivation cannot obtain enough oxygen. The decrease of pH value and oxygen content in the pit accelerates the dissolution of metals in the pit, which in turn promotes the inhalation and hydrolysis of CI ions. The above process repeats itself to form the autocatalytic process of the closed battery, and it is also the process that the metal in the pit is continuously dissolved. As shown in Figure 3.

Figure 3 The principle of pitting
(2) Crevice corrosion
Crevice corrosion is partial corrosion caused by the formation of concentration cells in the electrolyte, where the movement of related substances is blocked in very narrow gaps between surfaces of metals or non-metals. Crevice corrosion is a relatively common partial corrosion. For almost all metals and all media, if there is a stagnation gap, there will be crevice corrosion; passive metals are especially sensitive in neutral media containing active anions (CI and FR). The development process of crevice corrosion includes the lack of oxygen in the crevice, the hydrolysis of metal ions, the acidification of the solution, the destruction of the passivation film, the increase of the metal dissolution rate, and the autocatalytic process.
The principle of crevice corrosion is similar to that of pitting corrosion, both of which are caused by the autocatalytic effect of occluded cells. The oxygen content in the gap cannot be replenished in time due to the narrow gap, coupled with the migration of chloride ions and the hydrolysis of chloride; the pH value of the solution in the gap decreases, which further accelerates the dissolution of metals in the gap, as shown in Figure 4.

Figure 4 The principle of crevice corrosion
(3) Corrosion of oxygen concentration cells
Corrosion of oxygen concentration cells refers to a kind of corrosion behavior formed by the difference in oxygen. In an oxygen concentration cell, the region with a higher oxygen concentration on the metal surface has a higher potential and becomes the cathode of the corrosion cell. On the contrary, the region with a lower oxygen concentration on the metal surface has a lower potential and becomes the anode of the corrosion cell. The Nester equation describing the equilibrium potential in thermodynamics is used to explain the difference in potential when the metal is in contact with different concentrations of oxygen. The metal potential Ee1 in the oxygen-deficient region is lower, which becomes the anode region. The metal potential Ee2 in the oxygen-rich region is higher, which becomes the cathode region, as shown in Figure 5 (ignoring the ohmic potential drop in the solution).

Figure 5 Corrosion of metals in oxygen-deficient zone 1 and oxygen-enriched zone 2
Related News
- Installation of Main Bolts for Lap Joint Flange in High-Temperature Gas-Cooled Reactors
- Structural Design and Finite Element Analysis of Anchor Flanges
- Key Welding Technology for High-Neck Flange and Steel Pipe Joints
- The Design and Calculation of Stamped Lap Joint Flanges
- Development of Manufacturing Large Anchor Flanges
- Hardfacing the Inner Surface of Long-Neck Flanges Using CO₂ Gas-Shielded Welding
- UHV High-Neck Flange Welding
- Application of High-Neck Flange to UHV Steel Pipe Tower
- Analysis of the Cracking Cause of High-Neck Flanges
- Anchor Flanges for the East-West Gas Transmission Project
