Analyzing the Corrosion of Blind flanges
On this page
In the reforming unit of a continuous reforming device, the materials need to pass continuously through four reactors for the reaction. Each reactor has a heating furnace that provides the necessary reaction heat. Technologically, the four heating furnaces are combined into one to form a "four-in-one" square vertical heating furnace for reforming. The heating furnace pipes are U-shaped and arranged in a multi-pass parallel configuration, with the inlet and outlet of the furnace tubes connected by manifolds. The blind flange end of the manifold is sealed with a blind flange to facilitate engineering inspections and maintenance operations. This study begins with the observation of the corrosion on the blind flange of the outlet manifold of the four-in-one furnace in the reforming device.
During the overhaul, varying degrees of corrosion were found on the outlet manifold of the reforming unit of a petroleum refining enterprise, with the blind flange of the outlet manifold of the F702 furnace, which has the longest furnace tube, being the most severely corroded, as shown in Figure 1. The corrosion site is mainly concentrated on the lower right side of the blind flange end, and the corrosion morphology appears as vortex strips or pits. The material composition of the heating furnace includes 2% hydrogen sulfide (volume ratio, the same below), 1% hydrogen chloride, and 10% hydrogen; the flange is made from 1Cr5Mo; the blind flange end pressure of the manifold is 6,000–8,000MPa (with partial negative pressure); the temperature ranges from 520°C to 780°C; in recent years, the processing capacity has increased annually from 85.19 t/h to 168 t/h.
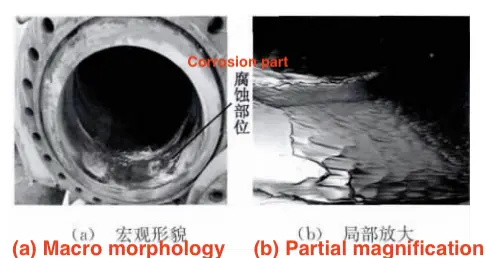
Figure 1 The corrosion morphology of outlet manifold blind flange

Figure 1 The corrosion morphology of outlet manifold blind flange
High-temperature sulfidation corrosion mainly occurs at the blind flange end due to elevated temperatures. Sulfide converts to elemental sulfur at 500°C, where the sulfur becomes more active. Elemental sulfur and metal undergo high-temperature sulfidation reactions. The raw oil used in the reforming unit is primarily high-sulfur crude oil. Sulfur corrosion has consistently had a severe impact on the equipment. The excessive sulfur content in the reforming raw oil is the primary cause of severe corrosion.
After sulfur corrosion, in a hydrogen-rich environment, atomic hydrogen can continuously invade the sulfide scale layer, making it loose and porous, allowing metal atoms and hydrogen sulfide to diffuse and penetrate each other, thereby perpetuating sulfur corrosion. On the other hand, hydrogen corrosion occurs when hydrogen atoms penetrate the metal at high temperatures to generate methane with carbon, but under the pressure at the blind flange end, hydrogen atoms can only carburize on the metal surface. Based on the working conditions of the manifold, it is evident that the heating furnace material contains a certain amount of hydrogen sulfide, with hydrogen accounting for 10%, indicating a hydrogen-rich environment, and the temperature at the blind flange end of the manifold has exceeded 500°C. In this case, high-temperature sulfur corrosion is inevitable, and the corrosion is accelerated in a hydrogen-rich environment. Therefore, hydrogen-hydrogen sulfide corrosion primarily occurs at the blind flange of the manifold.
After sulfur corrosion, in a hydrogen-rich environment, atomic hydrogen can continuously invade the sulfide scale layer, making it loose and porous, allowing metal atoms and hydrogen sulfide to diffuse and penetrate each other, thereby perpetuating sulfur corrosion. On the other hand, hydrogen corrosion occurs when hydrogen atoms penetrate the metal at high temperatures to generate methane with carbon, but under the pressure at the blind flange end, hydrogen atoms can only carburize on the metal surface. Based on the working conditions of the manifold, it is evident that the heating furnace material contains a certain amount of hydrogen sulfide, with hydrogen accounting for 10%, indicating a hydrogen-rich environment, and the temperature at the blind flange end of the manifold has exceeded 500°C. In this case, high-temperature sulfur corrosion is inevitable, and the corrosion is accelerated in a hydrogen-rich environment. Therefore, hydrogen-hydrogen sulfide corrosion primarily occurs at the blind flange of the manifold.
As the fluid moves, it continuously scours the metal surface and the loose, porous corrosion products from hydrogen-hydrogen sulfide chemical corrosion. As the processing volume increases, the fluid becomes turbulent at the blind flange end, and the increasing speed of the turbulent motion intensifies the scouring corrosion rate. Therefore, the corrosion of the blind flange is primarily a combination of chemical and physical corrosion. Severe physical corrosion accelerates the chemical corrosion rate. These two types of corrosion influence each other, as shown in Figure 2.
- Hydrogen-hydrogen sulfide chemical corrosion occurred on the blind flange of the outlet manifold, with its loose and porous corrosion products being scoured by high-velocity fluid.
- Excessive flow is the primary cause of corrosion on the blind flange of the outlet manifold.
- The clockwise flow of fluid in the outlet manifold, forming a star-shaped vortex, is why the actual corrosion takes the shape of a vortex strip.
- The maximum velocity and vortex occur at the lower right of the outlet manifold, which corresponds with the actual corrosion position.
- The improved flange cover structure effectively disrupts the clockwise flow pattern and reduces both the vortex and maximum fluid velocity at the actual corrosion location.
- The improved blind flange cover structure does not affect the medium flow rate of the furnace tube.
Related News
- Installation of Main Bolts for Lap Joint Flange in High-Temperature Gas-Cooled Reactors
- Structural Design and Finite Element Analysis of Anchor Flanges
- Key Welding Technology for High-Neck Flange and Steel Pipe Joints
- The Design and Calculation of Stamped Lap Joint Flanges
- Development of Manufacturing Large Anchor Flanges
- Hardfacing the Inner Surface of Long-Neck Flanges Using CO₂ Gas-Shielded Welding
- UHV High-Neck Flange Welding
- Application of High-Neck Flange to UHV Steel Pipe Tower
- Analysis of the Cracking Cause of High-Neck Flanges
- Anchor Flanges for the East-West Gas Transmission Project
